We strive to understand the biological mechanisms of hearing loss and then translate this knowledge to directly and rapidly improve the care of patients with hearing loss.
Rationale
A common clinical scenario is that a child is initially identified with a partial hearing loss, which then progresses to profound hearing loss over a period of months to years. Genetic defects are responsible for over half of these cases, however the specific mechanisms of how many of these mutations cause progressive sensorineural hearing loss is unclear. Right now, all we can tell a patient with hearing loss is that we know they have hearing loss, and that it is because of a problem in the cochlea. There are no more detailed tests available.
Methodology
Because of the difficulty in performing auditory research in humans, we study normal and transgenic mice that have hearing loss. We strive to perform comprehensive evaluation of the pathophysiology that creates the hearing loss. To do this, we develop novel technology to permit in vivo imaging and physiological measurements. We have created Volumetric Optical Coherence Tomography and Vibrometry (VOCTV) to non-invasively measure the vibratory patterns of intra-cochlear tissues. The level of detail within the cochlea that we can now image is roughly two orders of magnitude better than what is currently available with the latest MRI or CT techniques. Our goal is to be able to identify why any given patient that comes to clinic has hearing loss, and use this information to guide management using regenerative strategies that are in active development.
Examples
We are working now to translate this technology for use in humans. We expect that when this technique becomes available, doctors will be able to better diagnose and treat diseases of the middle and inner ear.
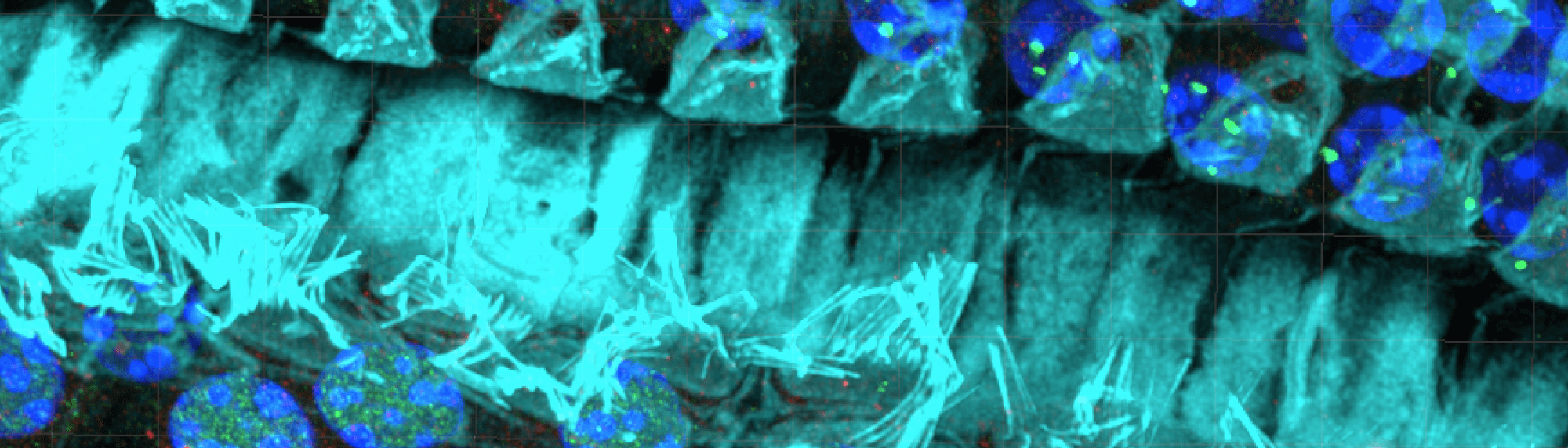
Inner Hair Cell – Auditory Nerve
We are studying how auditory neurons can be lost with aging or noise exposure. Below are videos which show one inner hair cell and multiple auditory nerve dendrites which connect to it.